Introduction of the Problem
Chlorofluorocarbons were hailed as miracle substances when they were first invented. “CFCs were hailed as the wonder chemical because they were useful and non-toxic” (Wile, 74). They allowed safe and cheap refrigeration, air-conditioning, industrial solvents, surgical sterilizers, and aerosols (Cooper). These uses saved thousands of lives. In 1985, however, a phenomenon known as the “ozone hole” was discovered. Quickly the scientific community decided that chlorofluorocarbons were to blame, and governments and interest groups throughout the world began working toward the eventual elimination of chlorofluorocarbon use. Nevertheless, these measures were too hasty, were based on faulty scientific assumptions and scanty research, and failed to consider the costs and consequences of CFC elimination.
Now, because of these restrictions, costs of refrigerants, solvents, sterilizers, and aerosols have soared. This has contributed to the food shortage in Africa and led to enormous costs to the refrigerant, air conditioning, and refrigerator industries, to higher prices to any users of industrial solvents, to less sterile operations, to higher prices of inhalers and fire extinguishers, and to many other unnecessary evils. “CFCs are the most efficient refrigerants, surgical sterilizers, and fire-fighting agents in the world. When they are completely banned, refrigeration, surgical sterilizers, and fire-fighting will be less efficient. As a result, people will die” (Wile, 73).
These problems may be solved simply, without much cost to the economy or environment. How? Remove the restrictions! They are unnecessary. The ozone “hole” is not as big or as dangerous as some would have us believe, and it is not a proven fact that CFCs cause it. However, there are tremendous costs because of this ban. “How will America pay for the ban on CFCs, which might cost $800 per person per year?” (Orient, “Costs and Benefits”)
Why then should the restrictions continue? There is no good reason for them to remain in place. We should remove the restrictions on chlorofluorocarbons because the restrictions are detrimental to the health of poor countries, to the strength of several industries, to the safety of certain segments of the American population, and they are also unnecessary in the light of scientific data concerning ozone depletion.
Policy Proposal
To eliminate the problem of high prices and less effective refrigerants and aerosols, the majority of the restrictions, bans, and special taxes on chlorofluorocarbons should be removed. The removal of these bans will allow producers to resume production of low price refrigerants and aerosols, will allow prices of refrigerant-containing products to come down, and will allow the best types of inhalers to be made again.
Who is responsible for the ban? Several international agreements were made regarding the elimination of CFCs, beginning with the Montreal Protocol in 1987. This pact set in motion the bans in place today. The Environmental Protection Agency (EPA) implemented these restrictions in the United States. The president or the head of the EPA could easily remove the ban.
The only way this could happen is if the specifics of chlorofluorocarbons become more widely understood: right now, 70% of Americans believe that CFCs should be banned because of their possibly damaging effect to the ozone layer. If, however, the majority thought that their good points outweigh any bad ones, the political leaders would take notice, and perhaps the media would as well. Nevertheless, at this time the somewhat biased coverage by the media, coupled with activists’ cries, has overwhelmed the more careful view of this matter.
The impact of these restrictions warrants immediate action towards their repeal. This is not a purely rhetorical issue, but a practical error on the part of those in charge of environmental and economic policies of our nation and of the world’s in general.
The Present View
CQ Researcher explains the present view of most people:
Beginning in the stratosphere at an altitude of about 15 miles and extending up into the mesosphere, the 25-mile-wide ozone layer protects Earth by blocking out most of the sun's harmful ultraviolet light. Breakdown of ozone by chlorofluorocarbons and other chemicals allows harmful radiation to reach Earth. 1. Oxygen molecules in the stratosphere are transformed into ozone by solar ultraviolet (UV) radiation, which splits the oxygen molecule (top) and releases highly reactive oxygen atoms. The free oxygen atoms then bind to oxygen molecules to form ozone molecules, which also are broken up by UV radiation. This continuous creation and destruction of oxygen and ozone occurs normally in the stratosphere. 2. Once certain chemicals, chiefly chlorofluorocarbons (CFCs), reach the ozone layer, UV radiation bombards the CFC molecule, breaking off an atom of chlorine. 3. The free chlorine atom attacks an ozone molecule, breaking off one of ozone's three oxygen atoms to form one chlorine monoxide molecule and leaving one oxygen molecule. 4. When the chlorine monoxide molecule encounters a free oxygen atom, produced during the natural mixing of oxygen and ozone (step 1), the oxygen atom breaks up the chlorine monoxide molecule and binds to its oxygen atom, forming a new oxygen molecule and leaving behind a free chlorine atom. 5. The newly freed chlorine atom can continue to destroy ozone molecules for many years (steps 3 and 4). Oxygen molecules continue to break apart and form ozone (step 1), but this natural replenishing process is slowed in the presence of chlorine monoxide. 6. Because oxygen, unlike ozone, does not reflect UV radiation, the sun's potentially harmful UV rays penetrate the depleted areas of the ozone layer and reach Earth's surface. (Cooper)
This UV light is then thought to injure people by damaging their skin and eyes.
This view has several flaws. First, there is a question regarding the mechanism by which the primarily northern hemisphere release of CFCs can counter the prevailing wind patterns and reach Antarctica. However, there is evidence, it is said, that CFCs have been found in large quantities over Antarctica. The mechanism by which CFCs give off free chlorine radicals is another difficulty of the idea. Some say that the UV light of the early spring breaks a chlorine atom free, though this seems to be open to criticism. However, those who blame CFCs for the ozone hole must accept this, for it is the only theory explaining how CFCs could harm the ozone layer.
Actually, CFCs themselves do not either act as a catalyst or react directly with the O 3. The mechanism, as described in the Merck Index (11th ed.) is this: ``Photodecomposition occurs in the stratosphere via absorption of UV radiation and subsequent release of atomic chlorine which can catalyze ozone breakdown.'' This hypothesis was developed by F.S. Rowland, President of AAAS, one of the foremost apologists for the ozone hole theory, and published in Nature 249:810 (1974).
How fast do the CFCs decompose? According to Elkins, et al, (Nature 364:780-793, 1993), the mean atmospheric lifetime of CFC-11 is 55 ± 8 years and of CFC-12 is 140 ± about 50 years. CFCs are so stable that they are actually being used as tracers to follow ocean currents.
In her book Environmental Overkill, Dixy Lee Ray asks: ``If freon breaks down and releases its chlorine in the stratosphere, what happens to the rest of the molecule?'' She states that no breakdown products of CFCs have ever been detected in the stratosphere, although at least 192 chemical reactions and another 48 photochemical reactions have been identified there. In his recent article, Elkins makes no mention of any CFC decomposition products. (Orient, “Ozone Eaters”)
This process of CFC breakdown into dangerous chlorine molecules has never been proven, as Jane Orient notes.
Nevertheless, these difficulties in the method by which CFCs may deplete the ozone layer is not the only trouble with the accepted ozone hole model. The “hole” itself is mostly a natural occurrence. There are only thirty or forty years of data on the stratospheric ozone levels over Antarctica. What is termed the “hole” is a yearly thinning of the ozone layer. This has occurred throughout the time for which measurements are available. During those forty years, the levels have remained similar for nearly every month. October showed the strongest decline, but few other months have shown any decline at all.
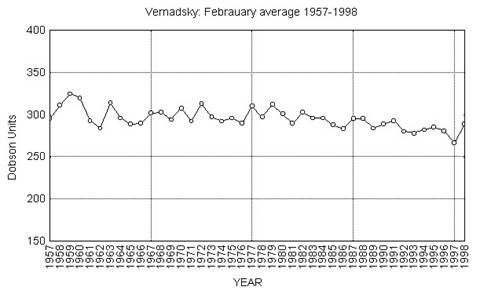


(Graphs from Yee.) (Dobson Units are the standard measure of ozone concentration in the upper atmosphere.) The February graph shows no steady decline in the ozone layer, but the October graph does. Even October’s decline, however, has not been steady, but a very erratic pattern of higher and lower readings.
The media primarily uses October in their various graphs of ozone depletion. Here is an example of a graph commonly used that includes data from during only one month, October, and which maximizes the trend by focusing on the minima and by chopping off the bottom part of the Y axis (Image from Newman).
The ozone hole is not really a hole, but a yearly weakening in the ozone layer due primarily to natural atmospheric conditions, specifically the strong polar vortex, very cold temperatures, and certain chlorine and nitrate reactions, with UV light powering the reactions.
Finally, and most importantly, the ozone hole has not been linked to any human or animal illness or death. The pundits have speculated on the dire consequences of ozone depletion, but no such disasters have appeared. Civil Defense Perspectives describes the conflicting reports used by one such doomsayer:
``Ultraviolet on the Increase''
``It is almost a truism: a loss of stratospheric ozone means there will be more harmful ultraviolet radiation (UV-B) at the Earth's surface,'' announced Paul Crutzen under this headline (Nature 356:104-105, 1992)… Contradicting his own headline, Crutzen observes that less ozone does not necessarily mean more UV-B. Sulfate particles, tropospheric ozone due to air pollution, and even cloudiness tend to decrease ultraviolet levels. Crutzen reports on a theoretical method of calculating UV-B levels. The maximum increase in dose in the Antarctic spring is estimated to be only 7% of the total typically received at the Equator. (Orient, “Hole in Theory”)
Increased radiation is the primary vehicle of death that they speculate about, but it has not yet caused any harm. Many have pointed to increasing skin cancer rates in Australia as a possible result of the ozone hole. Fred Singer explains how this is not the case:
I remember one Congressional hearing in 1987--there were so many-- where the witness was a noted dermatologist. He explained that since the hole had grown, since 1975, malignant melanoma had increased nearly 100% -- a frightening but true statistic. He simply did not explain three other facts to the Congress or to the media:
- An Antarctic hole should have no effect whatsoever on cancer rates in the U.S.
- In any case, melanomas have not been related directly to increased UV exposure.
- And finally, melanoma rates have been increasing by about 800% since statistics were first collected in 1935! There has been no corresponding change in the ozone layer or in the UV reaching sea level. To the contrary, measurements of UV-B (the biologically active component) have shown a pronounced and steady decline at every location; UV intensities in American cities are less today than in 1974. The causes of melanoma must include more than UV.
There does exist a correlation between UV-B intensity and benign skin tumors. Their frequency clearly increases as one approaches the equator, where the sun and the UV are both stronger, with tumor incidence more than doubling between the northern U.S. and south Texas . But we should not assume that all the increase is just due to UV intensities. Life styles in warmer climates are conducive to greater and longer exposures and may therefore contribute as much or more to skin tumors than the UV values themselves.
One other fact they don't much talk about: Since ozone content decreases strongly in moving towards the equator, a 5% decrease in the ozone layer, as calculated by some of the more pessimistic scenarios, would increase UV exposure to the same extent as moving about 60 miles south, the distance from New York to Trenton or from Palm Beach to Miami. An increase in altitude of 1000 feet would produce the same result. (Singer, “Experience”)
UV-A actual contributes far more to melanoma than does UV-B, as Singer notes:
90%-95% of melanomas are caused by UV-A (40). But UV-A is not absorbed by ozone at all, and therefore melanoma rates would not be affected by changes in stratospheric ozone. This important finding undercuts one of the main reasons for the Montreal Protocol and all subsequent regulations (41). (Singer, “Debacle”)
Thus, the purported evidence for illness because of the ozone hole has little real substance.


Besides, the ozone hole is limited to a practically uninhabited land. Because of the meteorological conditions required for the hole’s formation, this hole cannot expand much, if any, further. The polar vortex is the key to the hole. This wind pattern during the winter isolates the stratosphere over Antarctica, allows incredibly cold temperatures, pushes various chemicals including CFCs into the ozone layer, and continues long enough into spring to allow the hole to form for a few months before the pattern collapses. Interestingly, the “ozone hole” is always surrounded by a band with very high ozone concentrations, far higher than the rest of the world. This area of concentrated ozone filters into the former hole as soon as the polar vortex collapses. Note this picture of the ozone levels around Antarctica at the height of the “ozone hole,” October, and one month later: the hole in October quickly fills in, and the levels of ozone in Antarctica are then higher than anywhere else (pictures from Earth Probe TOMS Data & Images).
The evidence against chlorofluorocarbons is weak. The Montreal Protocol was an overreaction based on a public sentiment against the supposed ozone-destroyers. But the “ozone destroyers” have not had a major effect on the Antarctic ozone layer, and hole there is can not cause much harm. No other hole could form because meteorological phenomena preclude that possibility. The purported evidence for the hole’s threat, skin cancer rates, is little effected by the ozone hole. There is no good reason to restrict the use of CFCs.
Justification of Proposal
Cost-benefit Analysis
The benefits of CFC restrictions:
- The ozone hole may be 20% less deep than it was before.
Does this provide tangible benefits to people?
[T]he CFC ban will reduce the depth of the ozone hole (it won’t eliminate the ozone hole because it was there before CFCs were really popular) for four months of the year over a region of the world where very few people actually live. As a result, the ban on CFCs will not really save any lives. In fact, not a single malady has ever been linked to the “ozone hole,” so the elimination of CFCs will not even improve anyone’s life! (Wile, 73)
Thus, there are not any real benefits of restricting CFC use.
The costs of CFC restrictions:
- First of all there are the monetary costs. Civil Defense
Perspectives explains the money that will be spent:
How much are Americans willing to pay to avert an increased risk of skin cancer? How many skin cancers are they willing to endure for the sake of having air conditioning or a home refrigerator? How much should the Third World be required to pay in terms of foregone economic development to reduce the risk of skin cancer for palefaced Americans? And how much of a decrease in the yield of certain crops is a tolerable cost of refrigeration, without which 40% of the world's food would spoil?
The wire services, the AAAS, and environmental activists do not ask these questions. They simply assert that substitutes for CFCs can be found.
CFCs are widely used because they are stable, nonflammable, nontoxic, noncorrosive, and relatively inexpensive. They are excellent for fire extinguishers and insulation. Most importantly, they are found in 610 million refrigerators and freezers, 120 million cold storage units, 100 million refrigerated transports, and 150 million auto air conditioners. Replacing just the refrigerated food transportation vehicles would cost about $150 billion. The equipment would have to be scrapped; the compressors are designed to be used only with a certain type of freon.
Freon substitutes are under development but not in widespread production. They will be under patent (the patent on freon has expired), and the cheapest is about 30 times more costly than the freon-12 that it replaces. The substitutes are flammable and possibly carcinogenic. In addition, they are suspected of being greenhouse gases (Nature 344:513-516, 1990). The same political activists who are pressing for the ban on CFCs want halocarbons (HCFCs) to be banned by the year 2000 to prevent global warming. (Orient, “Costs and Benefits”)
- The physical costs are also large, as Dr. Wile states:
Fires will last longer before they are put out, resulting in loss of property and death. Surgical procedures will be less sterile, causing more infection, which will cause sickness and death. Finally, refrigerators will be so inefficient that third-world food distribution will be reduced, resulting in starvation! Not only is starvation due to poor food distribution a concern, but so is food spoilage. Even one of the big supporters of the CFC ban (Robert Watson) has admitted that “… probably more people would die from food poisoning as a consequence of inadequate refrigeration than would die from depleting ozone” (Environmental Overkill, Dixie Lee Ray, Regnery Gateway, 1993, p. 45). (73)
There are many good reasons to lift the bans. Dr. Jay Wile sums up this point: “When they [CFCs] are completely banned…people will die” (73). The ban is bad for the American people.
Conclusion
CFCs have been unnecessarily banned by an overreaction to new scientific findings. This ban has damaged some segments of the American economy, has led to increased fire-related deaths, and has contributed to the famines of Africa. The ban needs to be lifted! That would solve all of those problems, without a significant cost to the environment, as some have alleged. When we learn the truth regarding this problem, the solution becomes readily apparent. The American public needs to see past the misleading media reports, and to see the answer: remove the restrictions on CFCs.
Works Cited
Cooper, Mary. “Ozone Depletion.” CQ Researcher. 3 Apr. 1992 <http://80-library.cqpress.com.ezproxy.lib.ou.edu/cqresearcher/index.php>
Earth Probe TOMS Data & Images. 22 Nov. 2002 <http://toms.gsfc.nasa.gov/eptoms/ep.html>
Newman, Paul. “The Antarctic Ozone Hole.” Stratospheric Ozone: An Electronic Textbook. Image from Chapter 11 slides. 18 Nov. 2002 <http://see.gsfc.nasa.gov//edu/SEES/strat/class/Chap_11/11_slidz.htm>
Orient, Jane. “Costs and Benefits: the Ban on CFCs.” Civil Defense Perspectives. Mar. 1992: Vol. 8, No. 3. 17 Nov. 2002 <http://www.oism.org/cdp/V08_03.htm>
----“Is There a Hole in the Ozone-Hole Theory?” Civil Defense Perspectives. Mar. 1992: Vol. 8, No. 3. 17 Nov. 2002 <http://www.oism.org/cdp/V08_03.htm>
----“The Ozone Eaters are Falling.” Civil Defense Perspectives. Sep. 1993: Vol. 9, No. 6. 17 Nov. 2002 <http://www.oism.org/cdp/V09_06.htm>
Singer, Fred. “My Adventures in the Ozone Layer.” National Review. June 1989. 18 Nov. 2002 <http://www.sepp.org/ozone/advinozon.html>
----“The Ozone-CFC Debacle: Hasty Action, Shaky Science.” Technology: Journal of The Franklin Institute, Vol. 332A, pp. 61-66, 1995. 22 Nov. 2002 <http://www.sepp.org/ozone/ozonefranklin.html>
Wile, Jay. Exploring Creation with Physical Science. Anderson, IN: Apologia Educational Ministries, 2000.
Yee, Nicholas. The Ozone Scare. 16 Nov. 2002 <http://www.nickyee.com/ponder/ozone.html>







